





|

Strong,
weak and Electromagnetic forces
Nucleons
interact through three different means. These are refered
to a "forces" even though
"interactions" might be a more appropriate
term.
Strong
force: This is a short-range
attractive force. "Short range" refers
to the fact that the force dies off exponentially
in distance. This means that a nucleon is only
affected by the strong force of its nearest
neighbors. However, within that distance of less
than 2.0 E-15 m, it overwhelms the other
forces.
Weak
force: We will not concern ourselves
much with the weak force. It is much weaker and
much shorter range than the strong force. It is
principally of interest because it includes
interactions which can change neutrons to
protons. 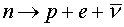 (a neutron
decays to a proton, an electron and an
anti-neutrino). This process is known as beta
decay. Since nuclei prefer to have roughly equal
numbers of neutrons and protons, beta decay
provides the avenue for nuclei to achieve the
optimal balance of nuetrons and protons. (a neutron
decays to a proton, an electron and an
anti-neutrino). This process is known as beta
decay. Since nuclei prefer to have roughly equal
numbers of neutrons and protons, beta decay
provides the avenue for nuclei to achieve the
optimal balance of nuetrons and protons.
Electromagnetic
force: The coulomb force, which acts
between protons, is a long-range force. For
nucleons which are near one another in the
nucleus, the coulomb interaction is dwarfed by
the strong force. But in large nuclei, two
protons at opposite ends of a nucleus continue to
feel the repulsive effects of the coulomb
interaction. The coulomb repulsion is responsible
for fission of large nuclei. If it were not for
the electromagnetic force, nuclei could grow
arbitrarily large.
Examples Nuclear
physics' index
|