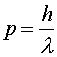Quantum properties of massive particles Just as light is usually thought of as a wave, electrons and other massive objects are usually thought of as particles. However, they have wave-like properties as well. A monoenergetic beam of electrons, neutrons or protons can make a diffraction pattern just like light. The wavelength of the beam is related to the momentum of the particles through the relation
The wavelength is known as the DeBroglie wavelength and is exactly the same relation as was used for photons. One could also use the relation E = hf, however the energy would include the rest energy, making the frequency so high that it does not enter the simple examples we will discuss here. In principle, all objects, no matter how massive, have DeBroglie wavelengths. However, for macroscopic objects, the wavelength is usually much smaller than the object's physical size and it is difficult to observe wave-like behavior. |