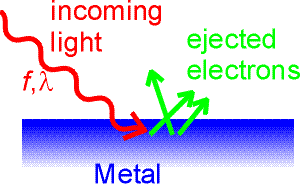
The photoelectric effect
In 1905 Einstein wrote three amazing papers. The paper expounding the theory of special relativity did not win him the Nobel prize (perhaps because of the timidness of the prize committee). Instead, it was his explanation of the photoelectric effect. The photoelectric effect refers to the interaction of light with electrons in a metal. Electrons are bound in the metal and cannot escape it without overcoming a potential energy W which is called the work function of the metal. Typically, work functions are a few eV. Light incident on the metal might give the electrons enough energy to escape the metal. The electrons are then collected and measured as a current.

An interesting aspect of the photoelectric effect is that the current stays practically zero for long-wavelength light, no matter how intense that light is. Einstein's explanation was that only photons with sufficiently high frequency (or sufficiently short wavelength) can give an electron enough energy to escape the metal. By adjusting the voltage on the metal to measure the kinetic energy of the ejected electrons, and by adjusting the frequency of the light, one can explore the expression E = hf.
The current begins only when the frequency of the incoming light corresponds to photons whose energy is sufficient to overcome the work function.
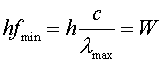
The min and max refer to the minimum and maximum values to create the current. Photoelectric experiments allow one to measure Planck's constant in a controlled experiment.
Practical examples: photons of ultraviolet light have enough energy to kill bacteria, and to cause sunburn; while photons of visible light generally do not. Photons of red light do not have enough energy to affect the the rods in our retinas that are used for vision in very dim light; so in very dim light, red objects appear black. Photons of red light also do not have enough energy to affect the kind of photographic materials that were used long ago; so for example red lips appear black in black+white photographs from the 19th century (unless the photos were retouched).