What is quantum physics?
The word quantum refers to discreteness. In Newtonian physics, all quantities are allowed to be continuous. For instance, particles can have any momentum and light can have any frequency. A quantum is a discrete packet of energy, charge, or any other quantity. For instance, one might say that electric charge is quantized in units of e. (Although with quarks, we will learn that it comes in units of e/3)
In the next few pages, we will discuss the fact that all exchanges of energy come in discrete amounts. For instance, when light is absorbed by some material, the energy of the material does not rise continuously, but in discrete jumps. One says that the material has absorbed a light quantum. We will also learn that energy levels (orbits) of an electron in an atom do not have a continuous range of possible energies, but instead that only discrete orbits are possible. This strange behavior is linked to the concept of wave-particle duality. We will see that particles can be described by wave functions that tell the probability of finding the particle.
A new fundamental constant must be introduced to account for all these new phenomena, Planck's constant. It is denoted by h.
![]()
This constant relates wave-like quantities to particle-like ones. For instance, a particle's energy E is related to the frequency f of its wave function, and a particle's momentum p is related to the wavelength l of its wave function.
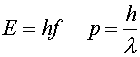
Quantum physics is necessary to understand the properties of solids, atoms, nuclei and light. In addition to the basis for understanding these natural phenomena, quantum principles represent a fundamental change in how humans view nature. To many philosophers, the probabilistic interpretation of quantum mechanics was a severe shock because of the change it required in older assumptions about determinism.