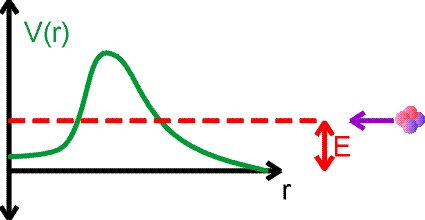
Nuclear decays and quantum-mechanical barrier penetration
Nuclei decay to lower energy states, emiting photons or other light particles which carry away the energy difference. Going to a lower energy state is like a ball rolling down hill, however for many nuclear examples, the half lives for reaching that lower states may be many years. This seems peculiar given that the characteristic times for particles to orbit the nucleus are 10-20 seconds. The reason that decays take such a long time have to do with quantum mechanics. We consider alpha decay using the figure below. Consider an alpha particle approaching a nucleus with energy E. The potential energy felt by the alpha is shown by the green curve.

The alpha particle could be caught and bound by the potential, except that it does not have sufficient energy to overcome the barrier. In nuclear physics, the repulsive part of the potential comes from the Coulomb repulsion, while the attracitve part of the potential is due to the strong force.
However, due to the quantum-mechanical nature of motion and the uncertainty principle, the alpha particle can tunnel through the barrier and get trapped by the potential. Of course, it could tunnel right back out. But tunneling may be very rare, both for entry and for exit. This is the case for long-lived decays like alpha decays or fissions. The decay of a nucleus via alpha decay can take a long time if the barrier is high and thick. Some nuclei, like lead, are considered stable but may have lifetimes greater than the age of the universe. If barriers are small, emission can occur arbitrarily fast.