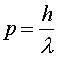
Quantum properties of massive particles
Light is often thought of as a wave; but it has particle-like properties also, since it is emitted and absorbed in discrete quanta that have well-defined energy and momentum. By the same token, electrons and other massive objects (the word massive in this context just means having a non-zero mass --- it doesn't have to be large) are often thought of as particles; but they have wave-like properties as well. For example, a monoenergetic beam of electrons, neutrons or protons can make a diffraction pattern just like light. The wavelength of the beam is related to the momentum of the particles by the relation
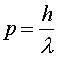
The wavelength l is known as the DeBroglie wavelength and it is given by exactly the same relation that was used for photons.
In principle, all objects, no matter how massive, have DeBroglie wavelengths. However, for macroscopic objects, the wavelength is so much smaller than the object's physical size that the wave-like behavior goes unnoticed.