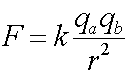
The Formula
Coulomb's law describes the force on one charged particle due to another charged particle.
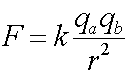
F is the force, qa and qb are the charges of particles a and b, r is the distance between the particles and k is a constant, 8.99x109 (Nm2/C2). You should ignore the signs of the charges qa and qb to calculate the magnitude of the force.
The direction of the force vector on each particle is toward the other if the one charge is positive and the other negative ("opposites attract"); or directly away from the other if both of the charges are positive or both negative ("like charges repel each other").
Note that the force falls off as the SQUARE of the distance, similarly to the behavior of the gravitational force. This fall-off with distance plays a key role in the classic demonstration of picking up uncharged bits of paper with a charged comb: The bits of paper have a net charge of zero, but the charges that are attracted to the comb are closer to the comb (and hence have smaller r and larger force) than the charges that are repelled; so the net force is toward the comb. The same effect is at work when you stick a balloon to an uncharged wall by first rubbing the balloon on a wool sweater.
If charge a
is in the presence of several charges b, c,... the force
that a
feels is the sum of the force vectors due to the remaining charges.
Like other vector addition problems, the total force is computed by adding
components.