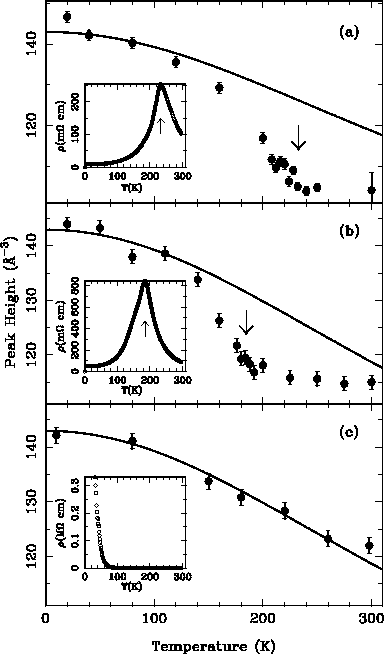
The arrows show the temperature of the metal-insulator transition. Sample (c) never becomes metallic, (a) does at 235K and (b) at 180K. What we can see is that the PDF peak height comes down sharply in the vicinity of the metal-insulator transition.
At the metal-insulator transition the Mn-O and O-O bonds are suddenly becoming more disordered and changing their lengths. This is exactly what is expected if polarons are forming: in the polaronic (above TMI) state there will be a range of Mn-O bond lengths on different sites depending on whether or not a charge resides there. In a non-polaronic state, all of the Mn sites will be identical, the Mn-O bond length distribution better ordered, and the PDF peaks sharper.
![[Postcard]](http://www.pa.msu.edu/people/thorpe/email.gif/) Return to
Simon Billinge:Home Page
Return to
Simon Billinge:Home Page