| (1) |
Coulomb's law
Force between two charges
The starting point in electrostatics is Coulomb's law,
which gives the force between two stationary charges,
| (1) |
Gravitational force law
| (2) |
Example
Find the ratio of the magnitudes of the gravitational and electrostatic forces between two protons.
Solution
The ratio of the magnitudes of the
gravitational and electrostatic forces is,
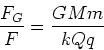 |
(3) |
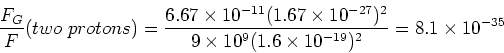 |
(4) |
Most charge is ``bound''
Due to the fact that the force between charges is
so large, most charge is bound. That means that negative
and positive charges are close to each other in regions
where the total charge is almost zero. This raises the
question. Why don't charges self destruct by crashing
into each other and annihilating? The answer is in quantum
mechanics, which provides an understanding of how
atoms are stable. Atoms are composed of electrons orbiting
the nuclei which contains the protons. This provides a
stable configuration of bound charges. Within electrostatics
bound charges still form dipoles. There are many more
charge dipoles than there are free charges. Many
electrostatic effects are due to dipoles rather than
free charges, so we need to understand dipoles well.
Force between many charges - superposition
Force on a charge q due to many other charges,
Q1, Q2, ...Qnis just the sum of the forces due to each of these charges, ie.
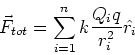 |
(5) |
Example- discrete charge distribution
Consider three charges located (and fixed) at (x,y) positions
![]() (in meters) and having charges
(in meters) and having charges
![]() .
Find the force acting on charge q3 due
to the other two charges.
.
Find the force acting on charge q3 due
to the other two charges.
Solution
We need to treat the force as a vector. In this problem
the best way to treat the force is to resolve the Coulomb
force between each pair of charges into its x-components
and it y-components. The charges form an isosceles right-angle
triangle, with charge q1 lying at the right-angle corner.
The other angles in the triangle are 45o.
The x-component of the force on charge q3 is given by,
| (6) |
| (7) |
The principle of superposition also applies when there is a continuous distribution of charge. For example charge distributions on rods, discs, spheres etc. However when treating these distibutions, the sum in Eq. (5) becomes an integral. In treating these problems, we define a small element of charge dQ. This is the amount of charge in a small part of the continuous charge distribution. We shall consider three cases:
- Lines: Then
![]() ,
where
,
where ![]() is the linear charge density.
is the linear charge density.
- Surfaces: Then
![]() ,
where
,
where ![]() is the surface charge density.
is the surface charge density.
- Volumes: Then
![]() ,
where
,
where ![]() is the volume charge density.
is the volume charge density.