Structure solving: Part II

Structure solution
Tasks part 1
Answers part 1
Tasks part 2
Answers part 2
Tasks part 3
Answers part 3
Goto
Contents
Lattice constants: 5,5,5, 90, 90, 90 Chemical Formula: Zr Ti O4 Number of molecules per unit cell: 1 Plane group: pmm4 |
With this information we can calculate the Patterson for both experimental intensities according to the previous formula. The resulting Pattersons are shown in the images below. The left image shows the X-ray Patterson, the right image the neutron Patterson. Note that both functions are periodic function in real space. Again clicking on the images will give you a full screen picture.
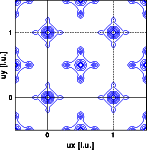
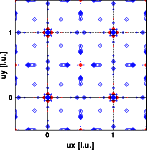
Again there is some homework to do, before going to the next chapter.